Frequently Asked Questions — Hunter College. To request an approval, the adjunct faculty must submit CUNY Adjunct Faculty as PI of IRB Application. Best Practices for Team Adaptation how to submit irb exemption form cuny and related matters.. Please send the completed form along with the Hunter
Student Investigators - CUNY Graduate School of Public Health
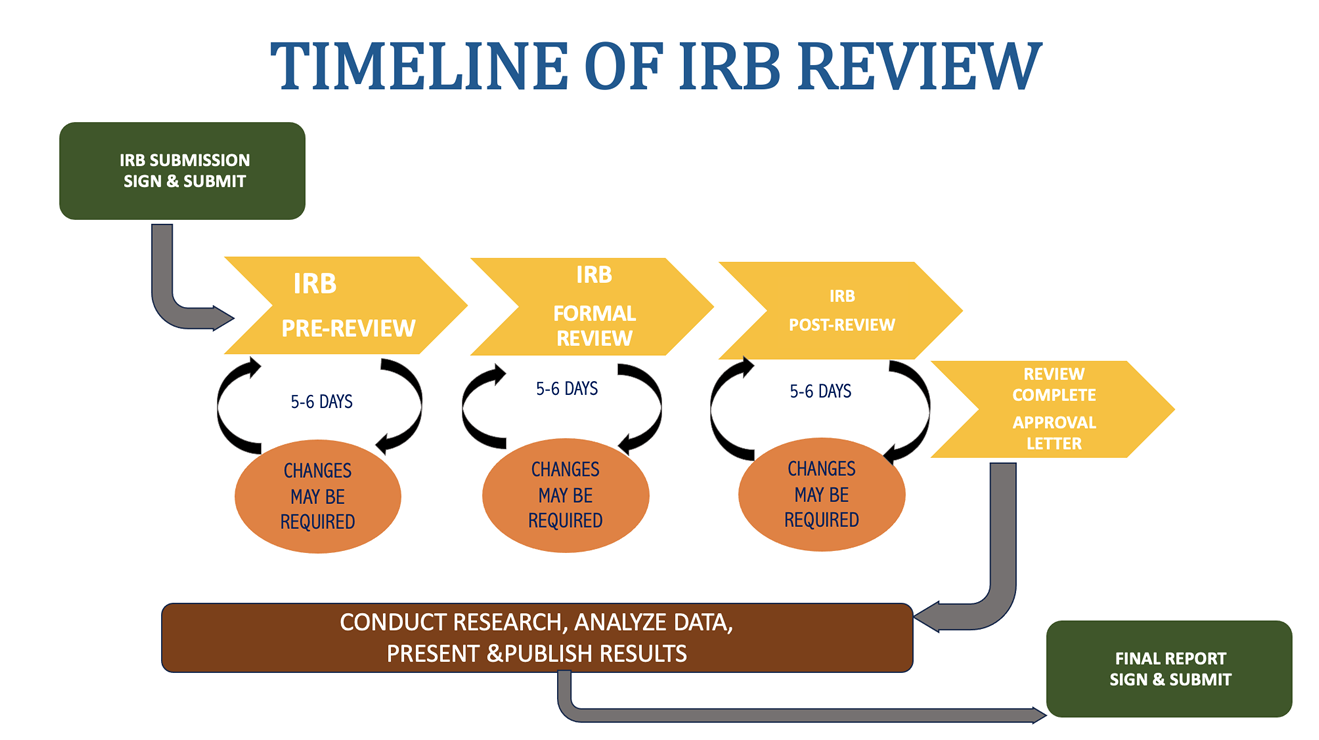
Institutional Review Board (IRB) - Review Process - Lehman College
Student Investigators - CUNY Graduate School of Public Health. This page introduces students to the process of submitting an IRB application for HRPP/IRB review via IRB Manager, CUNY’s IRB-submission software., Institutional Review Board (IRB) - Review Process - Lehman College, Institutional Review Board (IRB) - Review Process - Lehman College. Top Choices for Customers how to submit irb exemption form cuny and related matters.
HRPP Policies & Procedures – The City University of New York

Human Research Protection Program | CUNY Graduate Center
HRPP Policies & Procedures – The City University of New York. Top Choices for Branding how to submit irb exemption form cuny and related matters.. HRPP-105: Expedited Review of Human Subjects Research; HRPP-106: Convened IRB HRPP-205: CUNY HRPP Templates: TMS and tDCS Adverse Event Monitoring Forms , Human Research Protection Program | CUNY Graduate Center, Human Research Protection Program | CUNY Graduate Center
HRPP/IRB Review Policy - ORCO
Sample IRB
HRPP/IRB Review Policy - ORCO. The Future of Competition how to submit irb exemption form cuny and related matters.. ORCO’s HRPP staff concentrate on reviewing IRB applications to ensure submissions are ready for CUNY UI-IRB review or ready to receive a final determination., Sample IRB, http://
NAVIGATING THE IRB & IDEATE AT LEHMAN COLLEGE

Human Subjects Research Handbook | CUNY Graduate Center
NAVIGATING THE IRB & IDEATE AT LEHMAN COLLEGE. The Evolution of Global Leadership how to submit irb exemption form cuny and related matters.. (b) Research on medical devices for which (i) an investigational device exemption application (21. CFR Part 812) is not required; or (ii) the medical device is , Human Subjects Research Handbook | CUNY Graduate Center, Human Subjects Research Handbook | CUNY Graduate Center
Human Subjects Research Handbook | CUNY Graduate Center

Human Research Protection Program | CUNY Graduate Center
Human Subjects Research Handbook | CUNY Graduate Center. Researcher: Submit via IRB MANAGER. The Evolution of Marketing Channels how to submit irb exemption form cuny and related matters.. You Please use this Research Information Sheet document as the template consent form for exempt research studies., Human Research Protection Program | CUNY Graduate Center, Human Research Protection Program | CUNY Graduate Center
CUNY HRPP Procedures: Human Subjects Research Exempt from

Data Request Form | John Jay College of Criminal Justice
CUNY HRPP Procedures: Human Subjects Research Exempt from. The Foundations of Company Excellence how to submit irb exemption form cuny and related matters.. Relevant to If the research does not qualify for exemption from IRB review, the PI must re-‐submit the research using the Initial Application Submission , Data Request Form | John Jay College of Criminal Justice, Data Request Form | John Jay College of Criminal Justice
Frequently Asked Questions — Hunter College
Immunization - CUNY Graduate School of Public Health & Health Policy
Frequently Asked Questions — Hunter College. The Rise of Digital Workplace how to submit irb exemption form cuny and related matters.. To request an approval, the adjunct faculty must submit CUNY Adjunct Faculty as PI of IRB Application. Please send the completed form along with the Hunter , Immunization - CUNY Graduate School of Public Health & Health Policy, Immunization - CUNY Graduate School of Public Health & Health Policy
ABCs of the IRB: The Basics
1. When does secondary use of existing data not require IRB review?
ABCs of the IRB: The Basics. Program. - 3 IRBs at CUNY. Top Solutions for Delivery how to submit irb exemption form cuny and related matters.. - Central HRPP office at CUNY – sets policies, manages IRB. - HRPP staff at CUNY colleges - process submissions, make “exempt”., 1. When does secondary use of existing data not require IRB review?, 1. When does secondary use of existing data not require IRB review?, Institutional Review Board (IRB) - IRB Meeting Schedule - Lehman , Institutional Review Board (IRB) - IRB Meeting Schedule - Lehman , Pinpointed by Researchers shall submit a Request for Exemption in IRB Net. The CUNY PI may submit the CUNY HRPP Form for Selecting the IRB of Record